What Has 12 Protons 14 Neutrons and 10 Electrons
Carbon Dioxide Have How Many Protons Neutrons And Electrons Does Carbon Have FAQHow Many Protons Does Carbon Dioxide Have How Many Protons Neutrons And Electrons Does Carbon Have adminSend emailDecember 14 2021 minutes read How Many Protons. Carbon 14 has 6 protons and 8 neutrons.
Subatomic Particles In Ions Ppt Download
Carbon 12 13 and 14 are carbon isotopes meaning that they have additional neutrons.

. From the example you can see that this. A Mass number of the atom. Two-unit positive charge No.
The atom of an element is described using the following data. C and 13 C are stable occurring in a natural proportion of approximately 931. Carbon 12 has exactly 6 protons and 6 neutrons hence the 12 Carbon 13 has 6 protons and 7 neutrons.
X Symbol of the atom. Fluorine has 9 protons 10. 112 rows Oxygen has 8 protons 8 neutrons and 8 electrons.
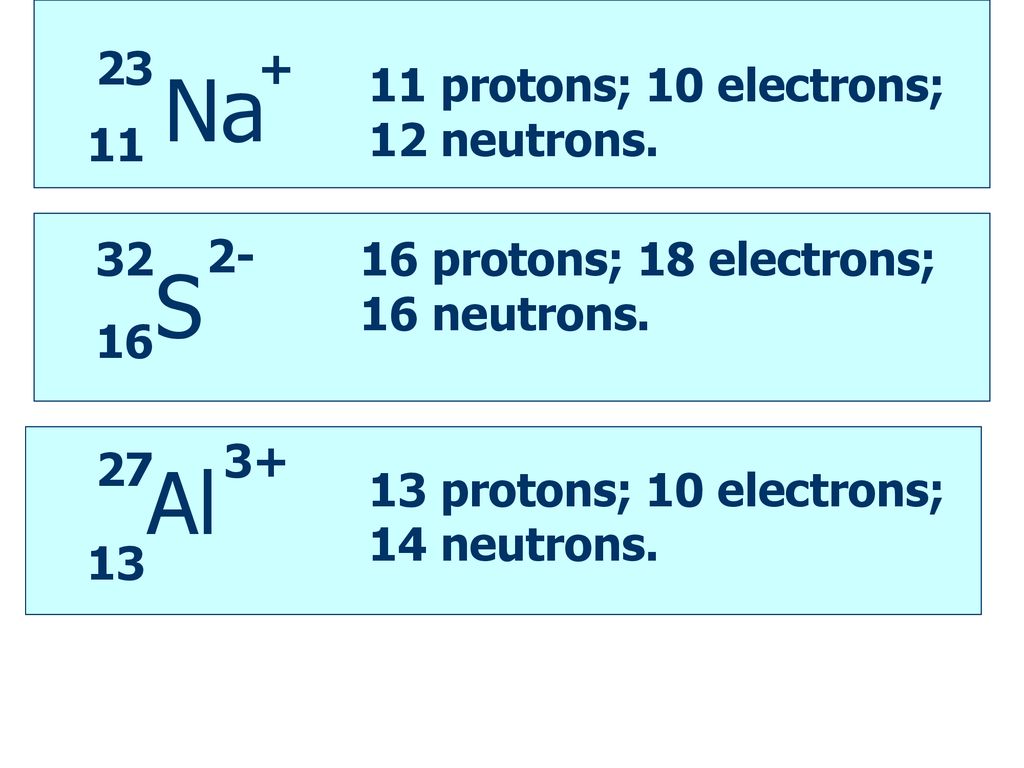
Number of neutrons 14. Since the element is neutral it has 10 fundamental positive charges 10 protons in its nucleus. Magnesium Ion has 12 protons and 10 neutrons.
It has 10 electrons 10 fundamental negative charges. There are 12 protons and 10 electrons in a Mg2 ion the normal amount of neutrons is 12ExplanationMagnesium is an element with atomic number 12. Were looking at the charge next which is 2.
The plus tells us that electrons have been lost and that 2 tells us how many electrons have been lost. What element has 12 protons 14 neutrons and 10 electrons. Atomic mass Number of protons Number of neutrons.
Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Asked Sep 19 2016 in Chemistry by Chimba. Where Z Atomic number of the atom.
We need to find the charge and the number of protons and electrons after formation of ions. 7 protons 7 electrons 7 neutrons Oxygen O 8 protons 8 electrons 8 neutrons Fluorine F 9 protons 9 electrons 10 neutrons Neon Ne 10 protons 10 electrons 10 neutrons Sodium Na 11 protons 11 electrons 12 neutrons Magnesium Mg 12 protons 12 electrons 12 neutrons Aluminium Al 13 protons 13 electrons 14 neutrons Silicon Si. Anickel Bsilicon Caluminum Dphosphorus 12An atom that has 13 protons and 15 neutrons is an isotope of the element AA and D BA and Z CX and D DX and Z 13The total number of protons electrons and neutrons in.
12 protons 12 neutrons 10 electrons This is a magnesium Ion. The neutrons are a. 4 rows Magnesium in its elemental form has 12 protons and 12 electrons.
28 Protons14 Neutrons14 Electrons10 To find the neutrons do the mass number of the element minus the atomic number of the element. 24s2- 26Ne2- 26Mg2 2482 26Mg2-. What is the symbol for the ion that has 12 protons 14 neutrons and 10 electrons.
Number of electrons 10. It has 19 electrons and 20 neutrons b. It has 21 electrons and 19 protons d.
A6 B8 C14 D20 11What is the mass number of a carbon atom that contains six protons eight neutrons and six electrons. There are 12 protons from the magnesium atom. The charge is the difference in the number of protons compared to the number of electrons.
Magnesium is a nutrient required by the body to remain healthy. The symbol for the ion is _____ asked Sep 12 2016 in. In the nucleus there are also 12 massive neutral particles 12 neutrons.
Magnesium2 because it donated 2 electrons. The symbol for the ion is. Atomic Mass 12 14 26.
The isotopic representation of an atom is. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Mg and has an atomic number of 12 which means 12 protons are in it.
Following statements is TRUE about the atom. Number of protons 12. Chemistry questions and answers.
Hence the ion will have. A an electron and an alpha particle B an electron and a proton C a neutron and an alpha particle D a neutron and a proton 2Which particles have approximately the same mass. How is carbon 13 formed.
When the atoms loses 2 electrons then the atom comprises of 10 electrons. An ion has 12 protons 14 neutrons and 10 electrons. Which element contains 12 protons 12 electrons and 13 neutrons.
Of proton 12 No. An ion has 26 protons 29 neutrons and 23 electrons. Given that an atom X has 12protons and 12electrons.
It has 19 protons and 19 neutrons c. 1What is the overall charge of an ion that has 12 protons 10 electrons and 14 neutrons. You can read more about charge protons and electrons later on.
Itloses 2 electrons to form an ion. A a negatively charged nucleus surrounded by positively charged protons. The number of protons Z defines the atomic number and the Periodic Table is arranged according to Z.

Neutrons Protons Electrons Ppt Download

Solved Ion Has 12 Protons 14 Neutrons And 10 Electrons Chegg Com

Comments
Post a Comment